Researchers have mapped the hidden control system of vision
For the first time, the smallest control system of vision in mammals has been mapped – a discovery that opens entirely new insights into how our vision works and how it can be affected by disease.
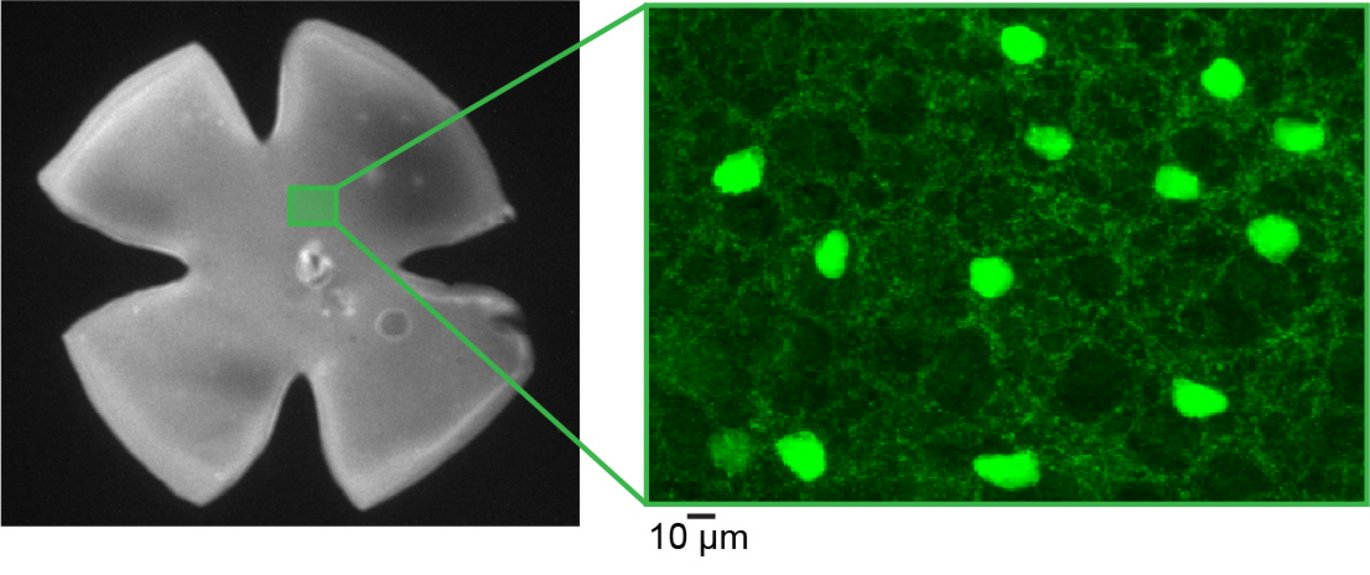
Vision is one of the most complex functions of our brain and requires a seamless interaction between many different brain structures to decode shapes, colours, depths, and movements and turn them into a meaningful whole.
Just like other brain functions, vision also depends on a balanced and controlled interaction between the chemical signals that "activate" and "brake" activity in the eye's cells – much like the accelerator and brake of a car. In research, the "brake" is known as GABA, which stands for gamma-aminobutyric acid, and it is the primary neurotransmitter that inhibits nerve activity and helps regulate the balance between excitatory and inhibitory signals in the brain.
Neuroscientific researchers have long been particularly interested in understanding how networks of excitatory and inhibitory neurons work together. It has been a fundamental challenge to uncover how these complex interactions in neural circuits create the precise and targeted actions that underlie both vision and other cognitive processes.
Together with colleagues from the USA, researchers from DANDRITE have, for the first time, conducted a comprehensive functional mapping of how the "brake" – inhibitory signaling – works in the part of the eye that processes and sends electrical signals to the brain, called the retina. It is in the retina that the first visual information is processed and then transmitted to the brain.
In total, the researchers mapped 44 different visual cells – some of which had never been described before. What was even more interesting was that these cells were characterized by a higher degree of systematic organization than previously known.
"Our result can be compared to finding a complicated map of an unknown landscape. Previously, we thought the landscape was randomly divided, but now we have mapped it, so we can see that each region has its own precise path and function," explains Professor Keisuke Yonehara, elaborating:
"By understanding how the different cells in the retina are organized and work in specific directions, we have revealed how the brain can precisely 'see' movement and orientation in our visual field – as if they have found the most efficient route through a complex network."
The key to this discovery was the development of a new GABA sensor, created by researchers in the USA, which allows for real-time observation and mapping of the precise activity of inhibitory neurons.
The research group's results have just been published in the prestigious journal Nature Neuroscience, and according to Keisuke Yonehara, this could be a breakthrough in understanding where and how certain eye disorders develop and occur.
"Many eye disorders are linked to imbalances in inhibitory signalling, including congenital nystagmus, where the eyes move involuntarily, quickly, and rhythmically. Now we have a 'map' that can give us deeper insights into this condition and hopefully also others related to this area."
Behind the research:
Study type: Basic Neuroscience Research
Collaborators: Danish Research Institute of Translational Neuroscience – DANDRITE, Nordic-EMBL Partnership for Molecular Medicine, Aarhus, Denmark, Department of Biomedicine, Aarhus University, Aarhus, Denmark, Department of Gene Function and Phenomics, National Institute of Genetics, Mishima, Japan, Graduate Institute for Advanced Studies, SOKENDAI, Hayama, Japan, Department of Neurosciences, University of California, San Diego, La Jolla, California, USA, Howard Hughes Medical Institute, University of California, San Diego, La Jolla, California, USA.
External funding: VELUX FONDEN Postdoctoral Ophthalmology Research Fellowship, The Food Science Institute Foundation, Narishige Neuroscience Research Foundation, Lundbeck Foundation, Novo Nordisk Foundation, European Research Council Starting, Chugai Foundation for Innovative Drug Discovery Science, Daiichi Sankyo Foundation of Life Science, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Mitsubishi Foundation, Toray Science Foundation, and Naito Foundation
Information on any impartiality issues: No conflict of interest
Direct link to the abstract or the scientific article: https://www.nature.com/articles/s41593-025-01935-0
Contact information
- Akihiro Matsumoto, National Institute of Genetics, Japan, aki.matsumoto@nig.ac.jp
- Keisuke Yonehara, Aarhus University, DANDRITE; National Institute of Genetics, Japan, Keisuke.yonehara@nig.ac.jp